博文
[转载]Dev Cell: 王佳谊/唐道林/于永春/周谦君团队揭示O-GlcNAc修饰型TR11B驱动肺癌恶性增殖的机制
||
肺癌是全球发病率和死亡率最高的恶性肿瘤,亦是严重危害我国人民健康及社会发展的重大公共卫生问题。O-GlcNAc糖基化修饰是一种重要的翻译后修饰(PTM),广泛存在于细胞核、细胞质蛋白的丝氨酸(Ser)或苏氨酸(Thr)上,参与肿瘤发生发展。然而,有关O-GlcNAc修饰相关的探究多局限于肿瘤细胞内部,有关其对肿瘤宏环境的调控机制尚不清楚。
2025年9月9日,上海交通大学医学院附属胸科医院王佳谊教授、周谦君教授及于永春教授,德克萨斯大学西南医学中心唐道林教授为共同通讯 在Developmental Cell上发表了题为A pathological role of O-GlcNAcylation-driven TR11B production and function in lung adenocarcinoma 的研究论文。上海交通大学医学院附属胸科医院博士研究生裘诗雨、检验科马丽芳副教授、病理科余科科副教授为本文共同第一作者。研究团队采用多种手段(组织微阵列芯片、多重免疫组织化学、细胞因子抗体芯片、多组学技术、免疫印迹),结合免疫功能正常或缺如小鼠荷瘤模型、小鼠自发成瘤模型,II型糖尿病小鼠尾静脉肺转移模型等,首次证实TR11B在肿瘤细胞内部发生了O-GlcNAc糖基化修饰,分泌至肿瘤细胞外部的O-GlcNAc修饰型TR11B可通过膜蛋白EHD1推动肿瘤细胞细胞周期,以促进其恶性增殖能力。
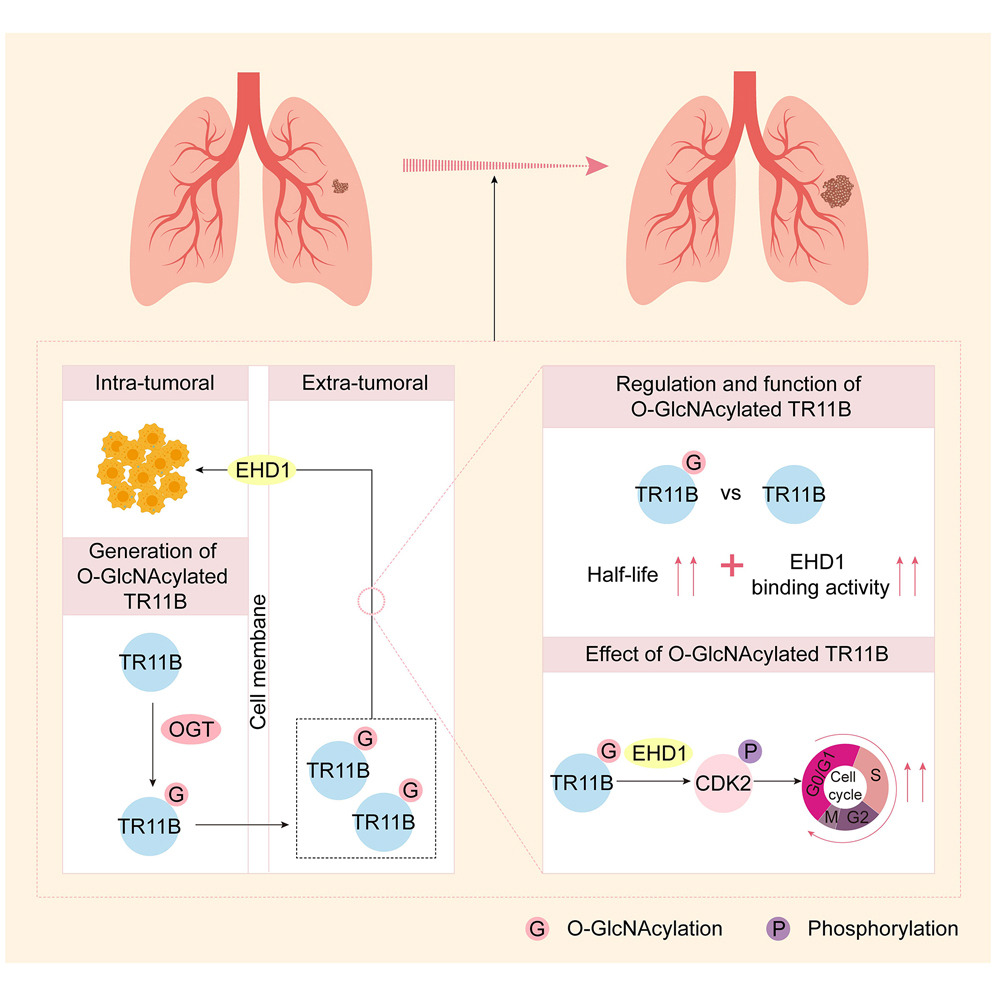
作者首先在肺腺癌肿瘤上皮细胞外部发现异常上调的O-GlcNAc修饰,该现象不仅在人、小鼠物种间保守,且可见于多种不同的胸部肿瘤类型,因此肿瘤外部的O-GlcNAc修饰极可能直接来源于肿瘤细胞的对外分泌。通过细胞因子抗体芯片锁定到TR11B、IL-4、CCL5,这三种细胞因子在外周血的水平受到O-GlcNAc修饰的正向调控。其中,TR11B不仅是受其调控变化最为显著的细胞因子,更是肿瘤上皮细胞外部O-GlcNAc修饰的重要来源。通过一系列体内、体外的回补实验证实TR11B处于OGT调控的下游。质谱发现,TR11B在S151位点发生O-GlcNAc修饰。经CHX追踪实验、TR11B互作蛋白质谱、泛素化实验、体外O-GlcNAc实验、分子对接、小鼠模型发现,TR11B蛋白S151位点O-GlcNAc修饰增强了TR11B蛋白稳定性、活性以及TR11B-EHD1互作强度,并以膜蛋白EHD1依赖的方式推动CDK2-Rb-E2F1细胞周期轴。
此外,该研究还深入探讨了这一发现的临床潜力。塞来昔布是FDA批准的骨关节炎一线用药。经机器学习预测塞来昔布可能具有潜在的OGT抑制作用。经研究发现,塞来昔布具有独立于COX-2抑制剂的O-GlcNAc修饰抑制功能,并在逆转化疗药物耐药、提高药物疗效、延长小鼠生存时间上具有亮眼表现,有望为临床治疗提供新的思路。
综上,该研究首次明确细胞因子TR11B、IL-4、CCL5发生了O-GlcNAc修饰,O-GlcNAc糖基化通过调控TR11B的功能和活性,可在外部视角重新调控恶性细胞的增殖能力。而打破上述O-GlcNAc修饰-细胞因子轴有望逆转化疗药物耐药的治疗困境,并提高肿瘤治疗效果。
摘要 Summary
Cytokines link inflammation to tumorigenesis, but the role of post-translational modifications in regulating their function within the extra-tumoral environment remains poorly defined. Here, we identify tumor-derived tumor necrosis factor (TNF) receptor superfamily member 11B (TR11B) as a key driver of lung adenocarcinoma (LUAD) progression and therapeutic resistance. Mechanistically, O-GlcNAc transferase (OGT)-mediated O-GlcNAcylation at serine 151 stabilizes TR11B and facilitates its interaction with the membrane protein EPS15 homology domain-containing protein 1 (EHD1), promoting cyclin dependent kinase 2 (CDK2) phosphorylation and cell cycle progression. Clinically, elevated O-GlcNAcylated TR11B correlates with advanced LUAD. Genetic deletion of Ogt suppresses tumor development in LUAD mouse models. Importantly, celecoxib, an U.S. Food and Drug Administration (FDA)-approved drug, inhibits O-GlcNAcylation and exerts antitumor effects. These findings reveal a pathological role for cytokine O-GlcNAcylation in LUAD and identify this axis as a potential therapeutic target.
亮点 Highlights
•TR11B is directly O-GlcNAcylated at serine 151 by OGT in tumor cells
•O-GlcNAcylation enhances TR11B stability and its interaction with EHD1
•Extra-tumoral TR11B promotes tumor progression by driving cell cycle progression
•Celecoxib acts as a potential O-GlcNAcylation inhibitor for LUAD treatment
DOI: https://doi.org/10.1016/j.devcel.2025.08.010
原文链接:https://www.sciencedirect.com/science/article/abs/pii/S1534580725005301
参考资料:Shiyu Qiu, Lifang Ma, Keke Yu, Xin Xu, Xiao Zhang, Wenjun Yu, Kai Wang, Xiaoting Tian, Yayou Miao, Yikun Wang, Wanxin Guo, Xiangfei Xue, Jiangtao Cui, Xuewen Yu, Rui Kang, Qianjun Zhou, Yongchun Yu, Daolin Tang, Jiayi Wang, A pathological role of O-GlcNAcylation-driven TR11B production and function in lung adenocarcinoma, Developmental Cell, 2025.
https://wap.sciencenet.cn/blog-446272-1502305.html
上一篇:STTT: 重庆医科大学揭示代谢酶PHGDH非经典RBP功能促进肝癌进展的新机制
下一篇:[转载]氨基己糖合成途径通过免疫检查点翻译延伸驱动肿瘤免疫逃逸新机制