精选
精选
科学家如何利用原子精度来强化化学反应
诸平
据《科技日报》(scitechdaily)网站2025年1月10日刊发来自中国科学院大连化学物理研究所{Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS)}提供的消息,科学家如何利用原子精度来强化化学反应(How Scientists Are Using Atomic Precision to Supercharge Chemistry)?
精确的纳米级双金属催化剂(bimetallic catalysts)工程使科学家能够通过微调电子结构来提高氢化性能。双金属粒子由贵金属和贱金属组合而成,具有独特的催化性能,使其对选择性非均相氢化反应非常有效。这些特性源于它们独特的几何和电子结构。为了使氢化既有效又有选择性,它需要在分子水平上进行特定的相互作用,催化剂上的活性原子精确地靶向底物中的官能团进行转化。
纳米工程与电子结构调谐(Nanoscale Engineering and Electronic Structure Tuning)
将这些粒子缩小到纳米级原子团簇或单原子合金进一步提高了它们的催化性能。这种尺寸的减小增加了表面分散,并优化了贵金属原子的使用。此外,这些纳米级的变化改变了活性位点的电子结构,这可以显著影响反应的活性和选择性。通过仔细调整贵金属单原子与基体金属之间的键合,研究人员可以创造灵活的环境,微调激活特定官能团所需的电子特性。尽管取得了这些进展,但实现这种活性位点的原子精确制造仍然是一个重大挑战。
原子位点调控的突破性研究(Breakthrough Study on Atomic Site Regulation)
中国科学院大连化学物理研究(Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China)所申文杰教授(Prof. Wenjie Shen)、李勇教授(Prof. Yong Li)与中国科学院中国科技大学(University of Science and Technology of China of CAS, Hefei, China)李微雪教授(Prof. Weixue Li)、德国卡尔斯鲁厄理工学院(Karlsruhe Institute of Technology, Eggenstein-Leopoldshafen, Germany)王跃民教授(Prof. Yuemin Wang音译)等人合作,成功调控了加氢反应活性位点的原子结构。相关研究结果于2025年1月3日已经在《化学》(Chem)杂志网站在线发表——Di Zhou, Junjun Wang, Minzhen Jian, Yong Li, Zheng Jiang, Shuang Liu, Yan Zhou, Jiake Wei, Christof Wöll, Wei-Xue Li, Yuemin Wang, Wenjie Shen. Fine-tuned coordination environment of Pt-Fe-Pt active site for selective heterogeneous hydrogenation of crotonaldehyde. Chem, 2025, 11: 102380. DOI: 10.1016/j.chempr.2024.11.018. Published 3 January 2025. https://doi.org/10.1016/j.chempr.2024.11.018
研究人员首先开发了一种方法,以Pt- Fe -Pt异质三聚体(Pt-Fe-Pt heterotrimer)的形式密集地填充和精确定位分离的Pt原子在α-Fe纳米颗粒上。Pt- Fe -Pt异质三聚体是通过H2还原Pt-Fe2O3粒子对得到的,其中3.3 nm的Pt粒子位于9.8 nm的Fe2O3粒子上。在H2还原过程中,氧化铁被还原为铁,通过表面合金化使Pt颗粒在铁颗粒表面分散成Pt- Fe -Pt异质三聚体。
增强氢化和途径发现(Enhanced Hydrogenation and Pathway Discovery)
此外,研究人员还揭示了Pt-Fe-Pt异质三聚体的形成途径和配位环境。在丁烯醛(crotonaldehyde简称CAL)气相加氢反应中,Pt-Fe-Pt异质三聚体更倾向于C=O键加氢生成丁烯醇(crotyl alcohol),而不是共轭C=C键。本征加氢速率提高了35倍,有效地解决了加氢反应中活性与选择性的权衡问题。
分子水平上对催化功能的洞察(Molecular-Level Insights into Catalytic Function)
此外,研究人员还发现了Pt-Fe-Pt异质三聚体的位点键识别模式。左端Pt原子锚定C=C键,中心Fe原子激活C=O键,C=O键被右端Pt原子吸附的H原子进一步氢化。

Fig. 2 Crotonaldehyde hydrogenation
申文杰教授说:“我们的研究在分子水平上量化了表面催化反应,并提供了一种以原子精度定制双金属催化剂活性位点的策略。”
这项研究得到了中国国家重点研发计划项目{National Key R&D Program of China (2022YFA1604103, 2021YFA1502802, and 2023YFA1508500)}、中国国家自然科学基金项目{National Natural Science Foundation of China (21573221, 21533009, and 22221003)}、德国科学基金会{Deutsche Forschungsgemeinschaft (DFG; 426888090–SFB1441)}、中国科学院战略重点研究计划项目{Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0450102)}以及量子科学技术创新计划项目{Innovation Program for Quantum Science and Technology (2021ZD0303302)}的资助。
上述介绍,仅供参考。欲了解更多信息,敬请注意浏览原文或者相关报道。
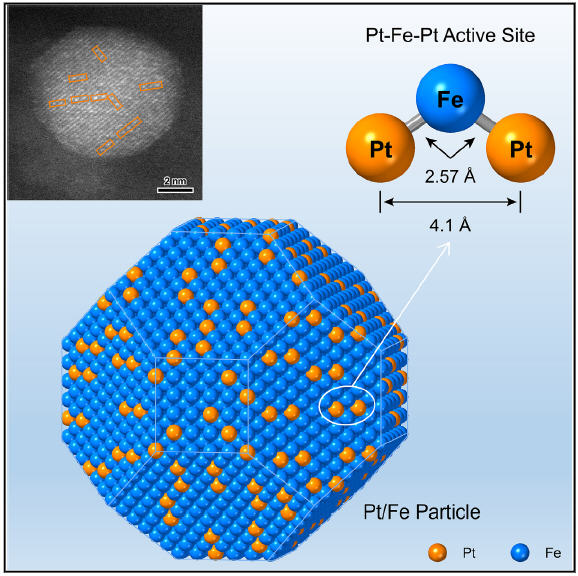
Densely populating and precisely arranging Pt atoms in the form of Pt-Fe-Pt over an Fe particle not only maximizes the utilization of the costly noble metal for catalysis but also enables the promotion of the overall reaction efficiency. The Pt-Fe-Pt heterotrimer catalyzes the preferential hydrogenation of C=O and simultaneously increases the reaction rate by 35-fold. The fine-tuned coordination environment of Pt-Fe-Pt allows the metal atoms to interact specifically with the functional groups in the reacting molecules and thereby circumvents the activity-selectivity tradeoff.
1)Coupling two Pt atoms with an Fe atom tunes the coordination environment of active site
2)Pt-Fe-Pt heterotrimer catalyzes preferential hydrogenation of C=O bond at high activity
3)Pt and Fe atoms act in a targeted and synergistic manner via site-bond recognition
Dispersing the catalytically more active noble metal at the single-site scale ensures maximum atom efficiency for selective heterogeneous hydrogenation over bimetallic particles. However, the low density and random location of the noble-metal atoms compromise the intrinsic activity and/or selectivity because of the resulting altered electronic structure. Here, we report that densely populating and precisely arranging Pt atoms in the form of a Pt-Fe-Pt heterotrimer not only catalyzes preferential hydrogenation of the C=O bond in crotonaldehyde (CAL) but also increases the reaction rate by 35-fold, circumventing the activity-selectivity trade-off. The Pt-Fe-Pt active site is fabricated by H2 reduction at 673 K of a Pt-Fe2O3 particle pair, wherein a 3.3 nm Pt particle sits on a 9.8 nm Fe2O3 particle. It interacts with the CAL molecule in a site-bond recognition manner: the left-end Pt atomanchors the C=C bond, whereas the central Fe atom activates the C=O bond, which is further hydrogenated by H atoms adsorbed on the right-end Pt atom.
转载本文请联系原作者获取授权,同时请注明本文来自诸平科学网博客。
链接地址:https://wap.sciencenet.cn/blog-212210-1468403.html?mobile=1
收藏