科学家证实了百年电子成键理论
诸平
Fig. 2 Using an X-ray diffractor to study the sigma bond. Credit: Yusuke Ishigaki
据日本北海道大学(Hokkaido University)2024年10月2日提供的消息,莱纳斯·鲍林是对的:科学家证实了百年电子成键理论(Linus Pauling Was Right: Scientists Confirm Century-Old Electron Bonding Theory)。
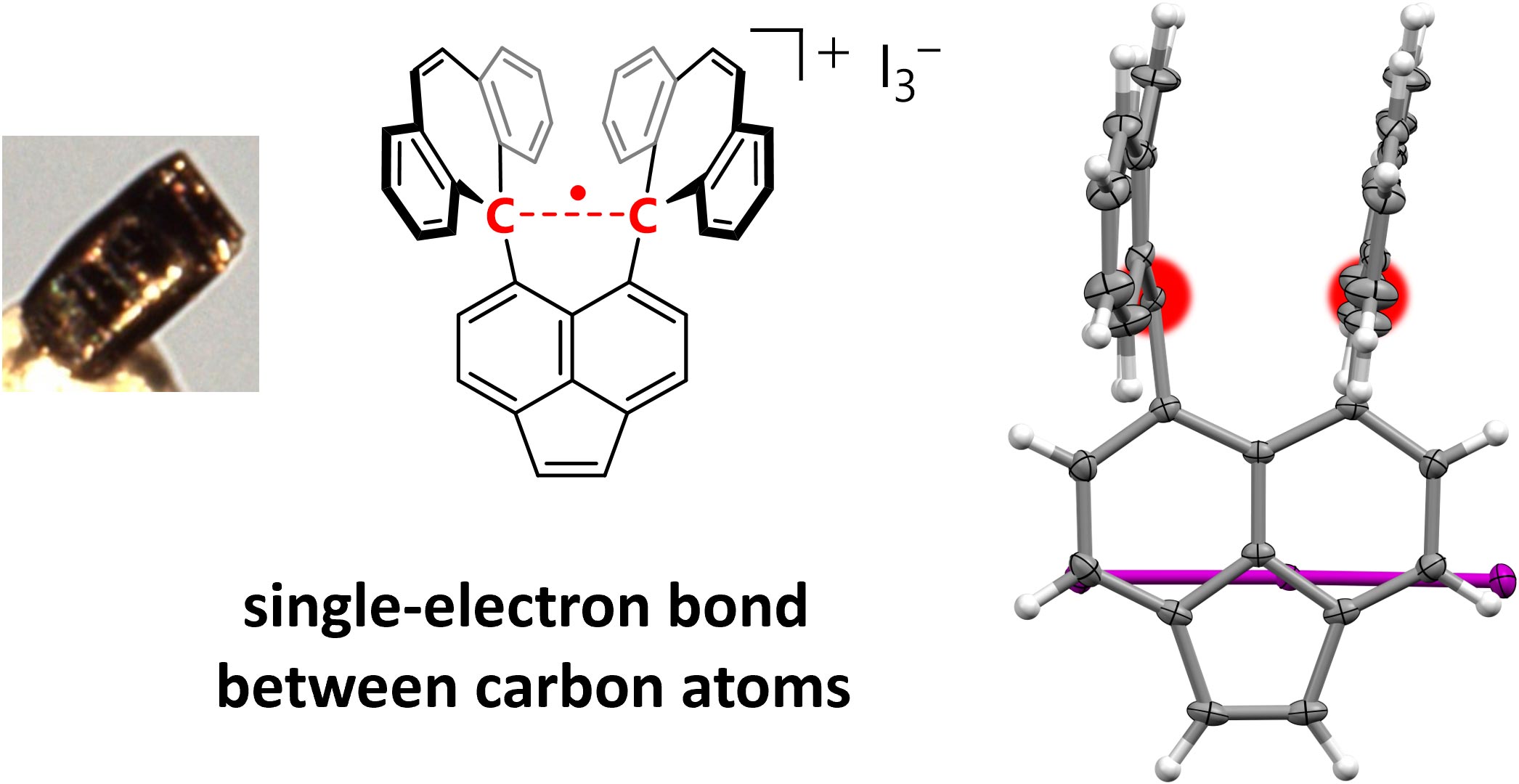
一项突破性的研究证实了两个碳原子之间存在稳定的单电子共价键,支持了莱纳斯·鲍林(Linus Pauling)20世纪初的理论,并为化学研究开辟了道路。
两个原子共用一对电子的共价键构成了大多数有机化合物的基础。1931年,诺贝尔奖得主莱纳斯·鲍林提出,由单个未配对电子形成的共价键可能存在,但这些单电子键可能比包含一对电子的标准共价键弱得多。从那以后,人们观察到了单电子键,但从未在碳或氢中观察到。寻找碳原子间共享的单电子键一直困扰着科学家们。
化学键的实验突破(Experimental Breakthrough in Chemical Bonding)
现在,来自北海道大学的一组研究人员已经分离出一种化合物,其中一个电子在两个碳原子之间以非常稳定的共价键共享,称为s键。他们的研究结果于2024年9月25日已经在《自然》(Nature)杂志网站在线发表——Takuya Shimajiri(島尻拓哉), Soki Kawaguchi(川口聡貴), Takanori Suzuki(鈴木孝紀), Yusuke Ishigaki(石垣侑祐). Direct evidence for a carbon–carbon one-electron σ-bond. Nature, Published: 25 September 2024. DOI: 10.1038/s41586-024-07965-1
参与此项研究的有来自日本東京大学大学院化学系(Department of Chemistry, Graduate School of Science, The University of Tokyo, Tokyo, Japan)、日本札幌北海道大学理学院化学系(Department of Chemistry, Faculty of Science, Hokkaido University, Sapporo, Japan)以及日本札幌北海道大学创意研究所(Creative Research Institution, Hokkaido University, Sapporo, Japan)的研究人员。
该研究论文的合著者、北海道大学化学系的石垣侑祐(Yusuke Ishigaki)教授解释说:“阐明两个碳原子之间的单电子s键的性质对于深入了解化学键理论至关重要,并将为化学反应提供进一步的见解。”
这个单电子键是通过六苯基乙烷(hexaphenylethane)的衍生物在碘的存在下进行氧化反应形成的,六苯基乙烷在两个碳原子之间含有一个非常伸展的共用单电子共价键(paired-electron covalent bond)。这个反应产生了深紫色的碘盐晶体。
研究小组使用X射线衍射分析来研究晶体,发现其中的碳原子非常靠近,这表明碳原子之间存在单电子共价键。然后他们用一种叫做拉曼光谱(Raman spectroscopy)的化学分析方法证实了这一点。
启示与未来研究(Implications and Future Research)
上述论文的第一作者、现供职于東京大学大学院化学系的島尻拓哉(Takuya Shimajiri)说:“这些结果因此构成了碳-碳单电子共价键存在的第一个实验证据,有望为这种很少被探索的成键类型之化学进一步发展铺平道路。”
本研究得到了孙正义育英财团(Masason Foundation)、日本文部科学省五星联盟研究计划{ Research Program ‘Five-star Alliance’ in ‘NJRC Mater. & Dev.’ of MEXT (Japan)}、丰田理研奖学金(Toyota Riken Scholarship)以及日本文部科学省-日本学術振興会科学研究经费{Grants-in-Aid from MEXT (JSPS Nos. 23K13726, 23K20275, 23K21107, and 23H04011) and JST PRESTO (No. JPMJPR23Q1)}的资助
上述介绍,仅供参考。欲了解更多信息,敬请注意浏览原文或者相关报道。
https://www2.sci.hokudai.ac.jp/faculty/wp/wp-content/uploads/2024/09/240926_pr.pdf
Covalent bonds share electron pairs between two atoms and make up the skeletons of most organic compounds in single, double and triple bonds. In contrast, examples of one-electron bonds remain scarce, most probably due to their intrinsic weakness1,2,3,4. Although several pioneering studies have reported one-electron bonds between heteroatoms, direct evidence for one-electron bonds between carbon atoms remains elusive. Here we report the isolation of a compound with a one-electron σ-bond between carbon atoms by means of the one-electron oxidation of a hydrocarbon with an elongated C–C single bond5,6. The presence of the C•C one-electron σ-bond (2.921(3) Å at 100 K) was confirmed experimentally by single-crystal X-ray diffraction analysis and Raman spectroscopy, and theoretically by density functional theory calculations. The results of this paper unequivocally demonstrate the existence of a C•C one-electron σ-bond, which was postulated nearly a century ago7, and can thus be expected to pave the way for further development in different areas of chemistry by probing the boundary between bonded and non-bonded states.
转载本文请联系原作者获取授权,同时请注明本文来自诸平科学网博客。
链接地址:https://wap.sciencenet.cn/blog-212210-1453687.html?mobile=1
收藏