博文
Two arrestin structures published on Nature
||
1. Crystal structure of pre-activated arrestin p44
Yong Ju Kim,Klaus Peter Hofmann,Oliver P. Ernst,Patrick Scheerer,Hui-Woog Choe& Martha E. Somme
nature 497,142–146 (02 May 2013)mdoi:10.1038/nature12133
Abstract
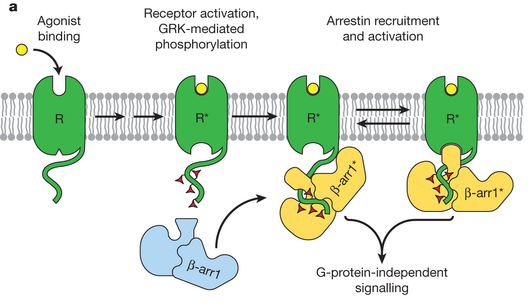
Arrestins interact with G-protein-coupled receptors (GPCRs) to block interaction with G proteins1, 2 and initiate G-protein-independent signalling3. Arrestins have a bi-lobed structure that is stabilized by a long carboxy-terminal tail (C-tail), and displacement of the C-tail by receptor-attached phosphates activates arrestins for binding active GPCRs4. Structures of the inactive state of arrestin are available5, 6, but it is not known how C-tail displacement activates arrestin for receptor coupling. Here we present a 3.0 Å crystal structure of the bovine arrestin-1 splice variant p44, in which the activation step is mimicked by C-tail truncation. The structure of this pre-activated arrestin is profoundly different from the basal state and gives insight into the activation mechanism. p44 displays breakage of the central polar core and other interlobe hydrogen-bond networks, leading to a ~21° rotation of the two lobes as compared to basal arrestin-1. Rearrangements in key receptor binding loops in the central crest region include the finger loop7, 8, 9, loop 139 (refs 8, 10, 11) and the sequence Asp 296 Asn 305 (or gate loop), here identified as controlling the polar core. We verified the role of these conformational alterations in arrestin activation and receptor binding by site-directed fluorescence spectroscopy. The data indicate a mechanism for arrestin activation in which C-tail displacement releases critical central-crest loops from restricted to extended receptor-interacting conformations. In parallel, increased flexibility between the two lobes facilitates a proper fitting of arrestin to the active receptor surface. Our results provide a snapshot of an arrestin ready to bind the active receptor, and give an insight into the role of naturally occurring truncated arrestins in the visual system.
2. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide
Arun K. Shukla,Aashish Manglik,Andrew C. Kruse,Kunhong Xiao,Rosana I. Reis,Wei-Chou Tseng,Dean P. Staus,Daniel Hilger,Serdar Uysal,Li-Yin Huang,Marcin Paduch,Prachi Tripathi-Shukla,Akiko Koide,Shohei Koide,William I. Weis,Anthony A. Kossiakoff,Brian K. Kobilka& Robert J. Lefkowitz
Nature497,137–141(02 May 2013)doi:10.1038/nature12120
Abstract
he functions of G-protein-coupled receptors (GPCRs) are primarily mediated and modulated by three families of proteins: the heterotrimeric G proteins, the G-protein-coupled receptor kinases (GRKs) and the arrestins1. G proteins mediate activation of second-messenger-generating enzymes and other effectors, GRKs phosphorylate activated receptors2, and arrestins subsequently bind phosphorylated receptors and cause receptor desensitization3. Arrestins activated by interaction with phosphorylated receptors can also mediate G-protein-independent signalling by serving as adaptors to link receptors to numerous signalling pathways4. Despite their central role in regulation and signalling of GPCRs, a structural understanding of β-arrestin activation and interaction with GPCRs is still lacking. Here we report the crystal structure of β-arrestin-1 (also called arrestin-2) in complex with a fully phosphorylated 29-amino-acid carboxy-terminal peptide derived from the human V2 vasopressin receptor (V2Rpp). This peptide has previously been shown to functionally and conformationally activate β-arrestin-1 (ref. 5). To capture this active conformation, we used a conformationally selective synthetic antibody fragment (Fab30) that recognizes the phosphopeptide-activated state of β-arrestin-1. The structure of the β-arrestin-1–V2Rpp–Fab30 complex shows marked conformational differences in β-arrestin-1 compared to its inactive conformation. These include rotation of the amino- and carboxy-terminal domains relative to each other, and a major reorientation of the ‘lariat loop’ implicated in maintaining the inactive state of β-arrestin-1. These results reveal, at high resolution, a receptor-interacting interface on β-arrestin, and they indicate a potentially general molecular mechanism for activation of these multifunctional signalling and regulatory proteins.
https://wap.sciencenet.cn/blog-355217-713987.html
上一篇:First structure of Class B GPCR released
下一篇:Making connections in the eye