博文
The Beer-Lambert Law
|||
See originals:
1. http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Spectroscopy/Electronic_Spectroscopy/Electronic_Spectroscopy_Basics/The_Beer-Lambert_Law
2. https://terpconnect.umd.edu/~toh/models/BeersLaw.html
The absorbance of a solution
Absorbance, denoted as $A$, is defined as the common logarithm of the ratio of incident to transmitted light intensity through a solution.
For each wavelength of light passing through the spectrometer, the intensity of light passing through the reference cuvette is $I_0$, while the intensity of light passing through the sample cuvette is $I$. If $I$ is less than $I_0$, then the sample has absorbed some of the light (the reflection of the cuvette surface is neglected).
$A =log_{10}\dfrac{I_0}{I}$
$I_0$ is the incident light intensity, and $I$ is the transmitted light intensity. $A$, $I_0$ and $I$ are functions of wavelength.
Transmittance ($T$) is defined as $\dfrac{I}{I_0}$, hence $A = -log_{10} T$.
The Beer-Lambert Law
The Beer-Lambert Law, also known as the Lambert-Beer Law, Beer's law and Beer-Lambert-Bouguer Law, states that the light absorbance is proportional to the length of the light path ($l$) and the concentration of the sample solution ($c$).
$A = log_{10}\dfrac{I_0}{I} = ϵlc$
$A$ is dimensionless. The proportionality constant, $ϵ$ - epsilon, is called molar absorptivity or molar extinction/absorption coefficient. It varies with substance type, wavelength, temperature, solvent, pH and other physichemical conditions. A substance with higher $ϵ$ at specific wavelength has more efficiency of absorbing light.
When $A = 0 (I_0= I)$, there is no light absorption at this wavelength; when $A = 1 (I = 0.1 I_0)$, 90% of the light at this wavelength has been absorbed.
Deviations from the Beer-Lambert Law
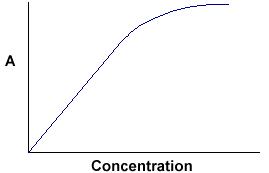
The fundermental requirement the Beer-Lambert Law is that every photon of light striking the detector must have an equal chance of absorption. That is, every photon must have the same $ϵ$ (monochromatic radiation), must pass througth the same path length $l$, and must experience the same absorber concentration $c$. The deviations from the Beer-Lambert Law are not actual failures of the law but rather the deviations caused either by failures of measuring instrument (apparent deviations) or by high concentration of the absorber (>0.01 M)(real deviations).
During the measurement, anything that upsets the fundemental requirements can lead to apparent deviations from the law. upset: to change the usual or expected state or order of something, especially in a way that stops it from happening or working. Most common apparent deviations from the Beer-Lambert Law can be due to: polychromatic radiation effect (polychromatic: involving or producing light in all colours) and unabsorbed stray light (杂光) errors.
Polychromatic radiation effect
The Beer-Lambert Law is observed only with truly monochromatic radiation. However, truly monochromatic radiation can never been produced by any light source. By using a very narrow exit slit (狭缝) on the monochromators, portions of the output from continuum light sources are isolated and monochromatic radiation is approximately obtained.
Every instrument (see spctrometer) has a finite spectral resolution, meaning that
When the solution of the sample is relatively concentrated, the Beer-Lambert Law is subjected to certain real deviations. These deviations are due to interactions between the absorbing species and alterations of the refractive index of the medium.
https://wap.sciencenet.cn/blog-3031432-964251.html
上一篇:LaTeX: subscripts and superscripts
下一篇:LaTeX: fractions
全部作者的其他最新博文
全部精选博文导读
相关博文
- • Minerals线下恳谈会:履践致远、与时偕行——对话中国科学院广州地球化学研究所期刊合作学者
- • 聚英才 建高地 | 北京理工大学“特立青年学者”全球招聘开启
- • 700年后日本或濒临灭绝?日本学者推算预测:届时或仅剩1名15岁以下孩子
- • [转载]【同位素视角】非英语母语学者如何区分’e.g.’, ‘i.e.’, ‘namely’与‘such as’等混淆难题
- • 美国佐治亚大学等机构学者:刈割策略对Bulldog 805紫花苜蓿+Tifton 85狗牙根混播草地产量及品质的影响
- • 美国堪萨斯州立大学、密苏里大学等机构学者研究成果:土壤水分管理策略和品种多样性对紫花苜蓿产量、营养品质和农场盈利能力的影