博文
SERS based aptasensor for acute kidney injury
|
SERS based Y-shaped aptasensor for early diagnosis of acute kidney injury
Dan Li【李丹】,‡ab Linlu Zhao【赵琳璐】,‡b Jin Qian【钱进】,b Heng Liu【刘恒】,b Jinmao You【尤进茂】,*a Ziyi Cheng【程子译】*b and Fabiao Yu【于法标】*b
RSC Advances, 2022, 12, 15910 - 15917 https://doi.org/10.1039/d2ra02813aConsidering the pivotal role of biomarkers in plasma, the development of biomarker specific sensing platform is of great significance to achieving accurate diagnosis and monitoring of the occurrence and progress in acute kidney injury (AKI). In this paper, we developed a promising surface-enhanced Raman scattering-based aptasensor for duplex detection of two protein biomarkers in AKI. Exploiting the base-pairing specificity of nucleic acid to form a Y-shaped self-assembled aptasensor, the MGITC labelled gold nanoparticles will be attached to the surface of magnetic beads. In the existence of specific AKI-related biomarkers, the gold nanoparticles will detach from magnetic beads into the supernatant, thus leading to SERS signal increasing, which can be used for the highly sensitive analysis of target biomarkers. In addition, the limit of detection calculated for each biomarker indicates that the SERS-based aptasensor can well meet the detection requirement in clinical applications. At last, the generality of this sensor in the early diagnosis of AKI is confirmed by using rats model and spiked plasma samples. This sensing platform provides a facile and general route for sensitive SERS detection of AKI-related biomarkers, which offers great promising utility for in vitro and accurate practical bioassay in AKI early diagnosis.
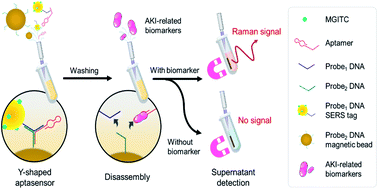
Figure 1 Schematic illustration of the SERS strategy for AKI biomarker detection.
Figure 2 Seed Growth and Characterization of AuNPs Nanoparticles from Diluted Seed Solutions. a) Absorption spectra of gold colloids, different growth processes, and absorption spectra of gold seeds at 520 nm. b) Dynamic light scattering map of AuNPs. c) TEM image of AuNPs, n=200. d) According to the particle history statistics in the image
Figure 3 Electrophoresis characteristics of the Y-shaped aptamer. Formation of Y-shaped aptasenor of NGAL (lane 1 - lane 3). Lane 1, 1 μM Probe1 + 0.3 μM NGAL aptamer; Lane 2, 0.3 μM Probe2 + 0.3 μM NGAL aptamer body; lane 3, 1 μM Probe1 + 1 μM Probe2 + 0.3 μM NGAL aptamer. Formation of Y-shaped aptasenor of Cys C (lane 4 - lane 6). Lane 4, 1 μM Probe1' + 0.3 μM Cys C aptamer; Lane 5, 1 μM Probe2' + 0.3 μM Cys C aptamer; lane 6, 1 μM Probe1' + 1 μM Probe2' + 0.3 μM Cys C aptamer.
Figure 4 Feasibility of the SERS-based aptamer sensor. a) Three groups of designed aptasensors with different hybridised DNA patterns, Solutions of the three samples were placed on a magnetic centrifuge to separate the supernatant and magnetic beads. c) UV-vis absorption intensities of supernatant from a) with the characteristic peak at 520 nm. TEM images of the Y-shaped aptasensor. c) before and d) after adding the targeted biomarker. Scale bar= 50 nm.
igure 5 Selectivity of aptasensor for NGAL and Cys C. Raman intensity at 1618 cm-1 of the NGAL (Cys C) aptasensor for the detection of Cys C, BSA, and NGAL (NGAL, BSA, and Cys C) with the concentration of 200 μg/mL. Each group of experiments was tested in parallel three times.
Figure 6 Quantitative SERS analysis of AKI biomarkers. Concentration-dependent SERS spectra of the aptasensor with concentrations of (a) NGAL ranging from 1 ng/mL to 10 ng/mL, and (b) Cys C ranging from 100 ng/mL to 1000 ng/mL. Corresponding calibration curves for series concentrations of the (c) NGAL and (d) Cys C solutions. Variations of the Raman intensity at 1618 cm−1 were used for quantitative analysis. The standard deviations were from three SERS measurements.
Figure 7 a) H&E staining of paraffin-embedded sections of rat kidneys after drug treatment. Scale bar, 50 μm. b,c) The concentrations of NGAL and Cys C in the blood of AKI rats at 4, 6, 8, 16, 24, and 48 hours were calculated from the standard calibration curve.
Table 1 SERS-based assay for NGAL, Cys C (n = 5 ).
Sample Number | Background (ng/mL) | Spiked (ng/mL) | SERS assay (ng/mL) | ELISA (ng/mL) | Recovery (%) | |||||
NGAL | Cys C | NGAL | Cys C | NGAL | Cys C | NGAL | Cys C | NGAL | Cys C | |
1 | 0.1 | 200 | 35 | 800 | 36.38 | 991 | 36.97 | 1013 | 103.66 | 98.88 |
2 | 0.1 | 200 | 45 | 900 | 41.12 | 1201 | 40.58 | 1156 | 91.16 | 111.22 |
3 | 0.1 | 200 | 25 | 700 | 28.93 | 904 | 29.53 | 889 | 115.32 | 100.57 |
4 | 0.1 | 200 | 40 | 800 | 40.12 | 973 | 40.59 | 1004 | 100.05 | 96.63 |
5 | 0.1 | 200 | 45 | 700 | 44.01 | 898 | 43.47 | 916 | 97.58 | 99.71 |
Conclusion
In summary, in this study, we successfully developed a duplex SERS-based aptasensing platform for the specific and sensitive detection of NGAL and Cys C biomarkers for the early and accurate AKI diagnosis. Highly selective SERS nanotags for NGAL and Cys C have been fabricated by functionalising Raman reporter-labelled AuNPs, integrated with magnetic spheres and specific aptamers by complementary base pairing to achieve activable biomarker detection using a recognition-release mechanism. The detailed sensing mechanism and feasibility of the designed nanoprobe in biomarker detection have been well validated by gel electrophoresis, DLS, and TEM. By testing the characteristic SERS signal change in 1618 cm-1, the designed aptasensors exhibited excellent selectivity to NGAL and Cys C to detect the supernatant collected upon magnetic separation and showed good quantitative analytical ability with LOD of 0.052 ng/mL and 0.34 ng/mL, respectively. Moreover, this aptasensor also showed good reproducibility and selectivity towards its targets in both rat models and spiked plasma samples. Compared with the traditional ELISA method, the SERS-based aptasensor could realise a comparable detection accuracy with a more extensive dynamic range from 10-2 to 105 ng/mL and less blood sample requirement of only 5 μL. It is believed that the constructed aptasensing platform could be further modified with other specific aptamers and thus easily extended for detecting a wide range of biomarkers to broaden its clinical applications.
https://wap.sciencenet.cn/blog-2438823-1340426.html
上一篇:Dual-targeted fluorescent probe or peroxynitrite
下一篇:[转载]《我的二舅》别人的二舅治好了我的精神内耗
全部作者的其他最新博文
- • Triphenylamine-AIE Materials for Cancer Theranostics
- • Fluorescent Probe forRatiometric Monitoring of Peroxynitrite
- • Fluorescence Probe for Pathological Stages of Wound Healing
- • Macrophage M2 polarization to neurological damage
- • SERS-RCA biosensor for profiling dual miRNAs
- • a glutathione-activated near-infrared fluorescent probe