博文
【JPCL】氮五环炸药结构稳定与储能机制:氢键、超氢键和反氢键耦合
||
Stabilization of the Dual-Aromatic Cyclo-N5ˉ Anion by Acidic Entrapment
Lei Zhang, Chuang Yao, Yi Yu, Sheng-Li Jiang, Chang Q Sun, Jun Chen
J. Phys. Chem. Lett. 2019, 10, 10, 2378-2385
https://doi.org/10.1021/acs.jpclett.9b01047 ![]() JPCL-5N.pdf
JPCL-5N.pdf
Pentazole anion, the best candidate for full-nitrogen energetic materials, can be isolated only from acidic solution for unclear reasons, which hinders the high-yield realization of a full-nitrogen substance with higher energy density. Herein, we report for the first time the discovery of the dual aromaticity (π and σ) of cyclo-N5–, which makes the anion unstable in nature but confers additional stability in acidic surroundings. In addition to the usual π-aromaticity, similar to that of the prototypical benzene, five lone pairs are delocalized in the equatorial plane of cyclo-N5–, forming additional σ-aromaticity. It is the compatible coexistence of the inter-lone-pair repulsion and inter-lone-pair attraction within the σ-aromatic system that makes the naked cyclo-N5– highly reactive to electrophiles and easily broken. Only in sufficiently acid solution can the cyclo-N5– become unsusceptible to the electrophilic attack and gain extra stability through the formation of hydrogen-bonded complex from surrounding electrophiles; otherwise, the cyclo-N5– cannot be productively isolated. The dual aromaticity discovered in cyclo-N5– is expected to be universal for pnictogen five-membered ring systems.

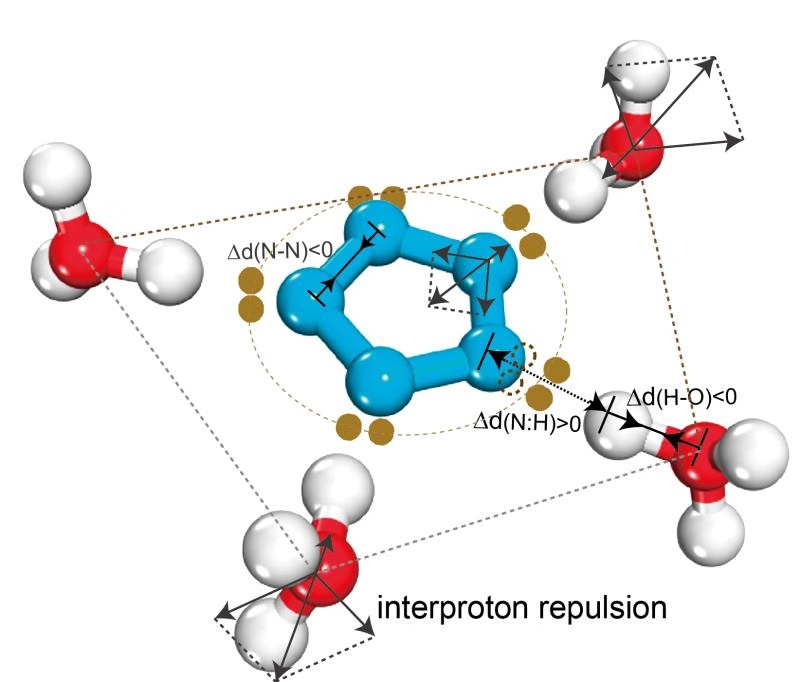
N sp2轨道杂化:环内N:=:N孤对(超氢键)排斥使其失稳,所以五环仅在富质子酸性环境中稳定;
3H-3H 排斥(反氢键)拉伸N:H-O氢键使H-O缩短并弱化N:=:N排斥并导致N-N共价键缩短,储能。
Ref:
Jiang, C. et al. Response to Comment on "Synthesis and characterization of the pentazolate anion cyclo-N5− in (N5)6(H3O)3(NH4)4Cl.". Science 359, eaas8953 (2018).
Zhang, C., Sun, C., Hu, B., Yu, C. & Lu, M. Synthesis and characterization of the pentazolate anion cyclo-N5− in (N5)6(H3O)3(NH4)4Cl. Science 355, 374 (2017).
https://wap.sciencenet.cn/blog-73032-1209819.html
上一篇:统一低配位原子、缺陷、表面、纳米结构属性的BOLS-NEP 理论(open access)
下一篇:亚纳米水滴的外壳壁厚和水合离子团簇体积的谱学计量